
Treat your cold sores with XERESE
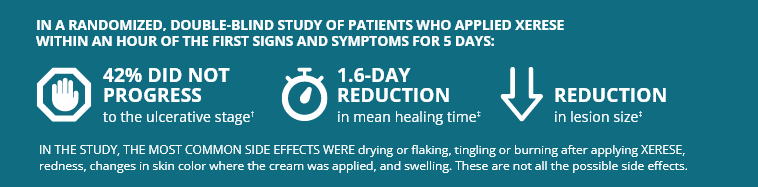
†Compared to 26% with placebo. Randomized study of 1,443 treated patients.
‡When compared with Placebo. Randomized study of 1,443 treated patients.
Talk to your healthcare provider if your cold sore is not better in 2 weeks.
Indication
XERESE® (acyclovir and hydrocortisone) cream 5%/1% is a prescription medicine used in patients ages 6 and older to lessen the healing time of cold sores (herpes labialis) and lessen the chance of a cold sore becoming worse (ulcerating). It should be used early, at the first sign of a cold sore.
Important safety information
- XERESE is for cold sores on lips and around the mouth only and should not be used in eyes, mouth, nose or on genitals. Use exactly as directed by your healthcare provider.
- XERESE is not a cure for cold sores.
- It is not known if XERESE is safe for or works in children younger than 6 years old.
- The safety of XERESE is unknown in women who are pregnant or breast-feeding, or in patients with a weak immune system (become sick very easily). Tell your healthcare provider about these or any other medical conditions you have before using XERESE.
- Do not cover the cold sore or the area around the cold sore with a bandage.
- Do not use other skin products (such as make-up, sun screen or lip balm) or other skin medicine on the cold sore or the area around the cold sore.
- Talk to your healthcare provider if your cold sore is not better in 2 weeks.
- The most common side effects of XERESE are: drying or flaking of the skin, tingling or burning after applying it, redness of the skin and swelling or changes in skin color where the cream is applied. These are not all the possible side effects of XERESE. Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Click here for full Prescribing Information.
INDICATION
XERESE® (acyclovir and hydrocortisone) cream 5%/1% is a prescription medicine used in patients ages 6 and older to lessen the healing time of cold sores (herpes labialis) and lessen the chance of a cold sore becoming worse (ulcerating). It should be used early, at the first sign of a cold sore.
Important safety information
- XERESE is for cold sores on lips and around the mouth only and should not be used in eyes, mouth, nose or on genitals. Use exactly as directed by your healthcare provider.
- XERESE is not a cure for cold sores.
- It is not known if XERESE is safe for or works in children younger than 6 years old.
- The safety of XERESE is unknown in women who are pregnant or breast-feeding, or in patients with a weak immune system (become sick very easily). Tell your healthcare provider about these or any other medical conditions you have before using XERESE.
- Do not cover the cold sore or the area around the cold sore with a bandage.
- Do not use other skin products (such as make-up, sun screen or lip balm) or other skin medicine on the cold sore or the area around the cold sore.
- Talk to your healthcare provider if your cold sore is not better in 2 weeks.
- The most common side effects of XERESE are: drying or flaking of the skin, tingling or burning after applying it, redness of the skin and swelling or changes in skin color where the cream is applied. These are not all the possible side effects of XERESE. Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Click here for full Prescribing Information.

